Main Article Content
Abstract
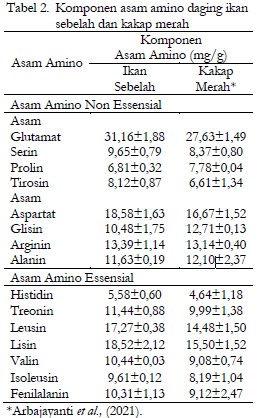
Trypsin is a proteolytic enzyme that plays a crucial role in protein digestion in fish. This study investigates the trypsin activity from the pyloric caeca of two fish species, flounder (Pseudorhombus sp.) and red snapper (Lutjanus sp.), and explores its relationship with the chemical composition and amino acid content of fish muscle. Crude enzyme extracts were analyzed for specific trypsin activity, while proximate composition was determined through measurements of protein, fat, moisture, ash, and carbohydrate content. Amino acid profiles were also evaluated to identify essential and non-essential amino acids in both species. Results showed that flounder had higher levels of protein (20.74 ± 0.46%) and ash (1.58 ± 0.13%) compared to red snapper, while its fat (0.45 ± 0.24%), moisture (76.54 ± 0.44%), and carbohydrate (0.69 ± 0.13%) contents were lower. Glutamic acid was the dominant non-essential amino acid in both species, with a higher concentration in flounder. Lysine was the most abundant essential amino acid in flounder (18.52 ± 2.12 mg/g), whereas histidine had the lowest concentration (5.58 ± 0.60 mg/g). The specific activity of trypsin was higher in red snapper (0.8240 U/mg) than in flounder (0.1127 U/mg), although both were lower than the trypsin activity reported in several other fish species. This study provides valuable insights into the enzymatic properties of trypsin and the nutritional composition of flounder and red snapper, highlighting their potential as sources of functional biomolecules for industrial applications.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.